TriLink's patented CleanCap® Reagent M6 enables the co-transcriptional capping of mRNAs by in vitro transcription (IVT) efficiently, producing mRNAs that have superior in vivo activity compared to legacy capping methods. Early studiesshowed that this innovative product increases mRNA expression by 30% or more versus enzymatic capping methods and is estimated to reduce manufacturing costs by 20-40%.
This blog discusses a recent publication (Mandell & Ujita et al.) reporting that CleanCap M6 resists enzyme-mediated decapping (Wurm and Sprangers) in vitro and that this decreased susceptibility to decapping correlated with substantially increased protein expression in vivo compared to mRNAs capped using existing industry standards.
These important findings by Mandell & Ujita et al. are the result of a research collaboration between TriLink Biotechnologies and Jeff Coller’s lab at John Hopkins University. Since then, the two have formalized a collaboration between TriLink Biotechnologies, a Maravai LifeSciences company, and the newly established Johns Hopkins University RNA Innovation Center. Jeff Coller, Bloomberg Distinguished Professor of RNA Biology and Therapeutics and a leader in mRNA stability and translation, is the director of the center and the corresponding author of the publication featured here.
Introduction to CleanCap M6 and other cap structures
The 5′ cap plays a crucial role in modulating mRNA translation and decay, helping to determine the fate of the transcribed mRNA once introduced into cells. Endogenous mammalian 5′ cap structures consist of an N7-methyl-G (m7G) residue attached to the first transcribed nucleotide via a 5′ to 5′ triphosphate linkage (Figure 1). The removal of the 5′ cap structure by the hydrolytic enzyme Dcp2 (Wurm and Sprangers) generates m7GDP and a 5′-monophosphate mRNA (Figure 1). After decapping, the 5′-phosphate mRNA is rapidly degraded in the 5′-3′ direction by the exonuclease Xrn1.
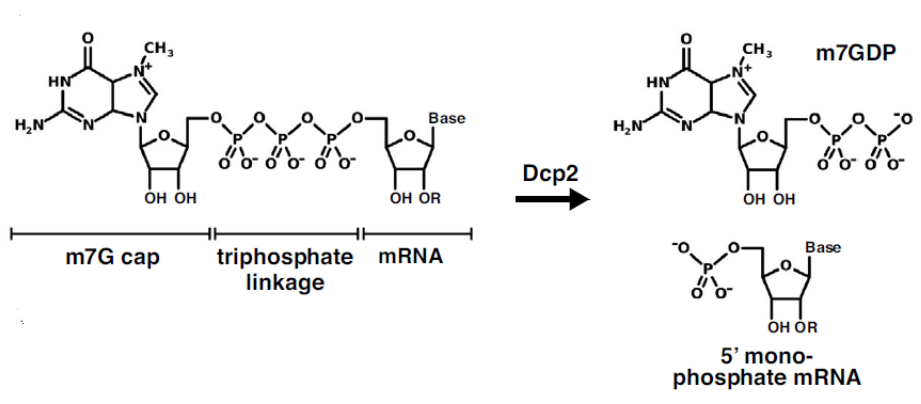
FIGURE 1. Structure of m7G capped mRNA. The Dcp2 mediated decapping reaction produces m7GDP and 5′-monophosphate mRNA. Taken from Wurm and Sprangers and used under CC BY-NC-ND 4.0 license.
3′-O-Methylation of the m7G ribose ensures that the cap is incorporated in the correct orientation (Stepinski et al.) during mRNA synthesis by in vitro transcription (IVT). In addition, the first and second transcribed nucleotides can be methylated at the 2’-O-ribose to yield Cap1 or Cap2 structures, respectively (Ramanathan et al.). These methylated sites are directly incorporated by co-transcription with corresponding trinucleotide analogs (Henderson et al.). Among various cap analogs, N6-methylated adenosine (m6A) at the first transcribed nucleotide of IVT mRNA received considerable attention because of its ability to increase protein expression through an inhibition of decapping (Mauer et al.). However, the effect of cap proximal m6A and 3′-O-methyl (3′OMe) on IVT mRNAs when utilized in conjunction was unclear.
To elucidate the effects of m6A and 3′OMe, and other cap structures, Mandell & Ujita et al. used a combination of in vivo, tissue culture, and in vitro biochemical approaches to comparatively study mRNAs co-transcriptionally capped with the four cap structures shown in Figure 2, namely, AG 3′OH, AG 3′OMe (commercially known as CleanCap® Reagent AG 3′OMe), M6 3′OH, and M6 3′OMe (commercially known as CleanCap® Reagent M6).

FIGURE 2.Schematic of 5′ cap structures studied by Mandell & Ujita et al. (2025).Image used under CC BY 4.0 license.
It is noteworthy that, using a design of experiments approach, numerous conditions including pH, nucleoside triphosphate, cap, and Mg2+ concentrations were screened to prepare mRNAs capped by these cap analogs. This systematic optimization allowed the identification of an IVT protocol that yields high quantities of >95% capped mRNA and produces minimal double-stranded RNA (dsRNA) by-products.
In vivocomparison of CleanCap M6 and other cap structures
To test the effect of the 5′ cap structures on protein expression in vivo, N1-methylpseudouridine (m1ψ)-modified luciferase (Fluc) mRNAs were encapsulated into lipid nanoparticles (LNPs) and tail vein-injected into groups of mice (N = 5). Fluc activity was then measured using whole-body bioluminescence imaging at various time points over 48 h. It was found that Fluc mRNAs capped using M6 3′OMe yielded 3.1-fold more total luminescence over 48 h than Fluc mRNAs capped using AG 3′OH, 1.5-fold over AG 3′OMe, and 1.9-fold over M6 3′OH (Figure 3).

FIGURE 3. Total 48-hr luminescence of mice injected with various 5′ capped Fluc mRNAs. Bars above each pair of treated mice show the p values of Mann-Whitney comparison. Taken from Mandell & Ujita et al. (2025) and used under CC BY 4.0 license.
In addition, serum was collected from each animal for cytokine and chemokine analysis at 3 h and 24 h post-injection, and no significant differences in the overall inflammatory profiles of the co-transcriptionally capped mRNAs were observed. Purifying mRNAs by reverse phase-high-performance liquid chromatography provided no observable change in protein expression or immune responses, confirming that the difference in potency was due to the cap structure itself.
Notably, M6 3′OMe mRNAs, delivered at a dose of 0.3 mg/kg, resulted in approximately the same level of expression as AG 3′OH mRNAs delivered at a higher dose of 1 mg/kg. To confirm that this effect was not Fluc specific, mRNAs encoding human erythropoietin (hEPO), a commonly used model for gene replacement therapies, were similarly tested in mice. Again, M6 3′OMe mRNAs yielded roughly 3-fold more hEPO protein at 24 h, corroborating the findings with Fluc. Together, these data demonstrate that the M6 3′OMe cap analog increases mRNA potency.
Translation initiation for CleanCap M6 and other cap structures
Protein expression of exogenous mRNA is a function of mRNA half-life and translation efficiency. Because translation initiation begins with eukaryotic translation initiation factor 4E (eIF4E) binding to the 5′ cap (Jackson et al.), Mandell & Ujita et al. investigated whether M6 3′OMe had a direct effect on translation initiation.
Purified human eIF4E was incubated with increasing concentrations of model RNA oligos capped with AG 3′OH or M6 3′OMe. Using quantitative gel shifts, the equilibrium dissociation constant (KD) of eIF4E for both capped oligos was measured. It was observed that M6 3′OMe reduces the affinity of eIF4E for the RNA relative to AG 3′OH by 3.5-fold.
To determine whether this impaired binding has a negative impact on translation initiation, M6 3′OMe or AG 3′OMe Fluc mRNAs were transfected into HEK293T cells. Initial luminescence changes were then measured, with the goal of measuring early translation and minimizing the impact of RNA decay. Despite a lower affinity for eIF4E, comparable rates of early translation initiation rates were measured from 30 min to 120 min post-transfection.
AG 3′OMe or M6 3′OMe capped Fluc mRNAs were also translated in cell-free rabbit reticulocyte lysates, which are deficient in catalytic mRNA decapping, and there was no statistical difference in expression.
Decapping and decay for CleanCap M6 and other cap structures
Given the lower affinity for eIF4E, Mandell & Ujita et al. considered whether impaired enzymatic decapping by Dcp2, and thus a potentially longer mRNA half-life, might be responsible for the higher protein expression seen with M6 3′OMe capped mRNAs.
To explore this possibility, capped RNA oligos were incubated with recombinant human Dcp2 enzyme, and the fraction of decapped RNA over time was quantified by liquid chromatography-mass spectrometry (Figure 4A). RNAs capped with AG 3′OMe and M6 3′OH were both decapped less efficiently than AG 3′OH. Moreover, combining the 3′OMe and m6A modifications in M6 3′OMe showed an additive effect with even greater resistance to Dcp2-mediated decapping.

FIGURE 4. Effect of cap structure on decapping. (A) Dcp2-mediated in vitrodecapping of 43-mer oligos. Error bars, mean ± standard deviation of three replicates. (B) Dcp2 + Xrn1 in vitro decay of 43-mer oligos. Error bars, mean ± standard deviation of three replicates. Taken from Mandell & Ujita et al. (2025)and used under CC BY 4.0 license.
Building on this system, Mandell & Ujita et al. reconstituted a minimal in vitro decay system, comprised of recombinant human Dcp2 decapping enzyme and commercially available yeast Xrn1 5′-3′ exonuclease. RNA oligos capped with either AG 3′OH or M6 3′OMe were treated with Dcp2 and Xrn1, and the time course for RNA decay was quantitated. In this decay system, M6 3′OMe increased the half-life of the RNA by approximately 2.5-fold (Figure 4B).
To assess whether M6 3′OMe impacts mRNA half-life in cells, full-length unmodified Fluc mRNAs capped with either M6 3′OMe or AG 3′OMe were transfected into HEK293T cells and mRNA decay over time quantified by quantitative reverse-transcription PCR (qRT-PCR). M6 3′OMe cap significantly increased the longevity of Fluc mRNA in HEK293T cells (up to 23-fold relative to AG 3′OMe), suggesting that increased resistance to decapping by M6 3′OMe can improve mRNA longevity.
Concluding comments
Mandell & Ujita et al. concluded that mRNAs capped with M6 3′OMe (CleanCap M6) yield significantly more protein in vivothan those capped with AG 3′OH, AG 3′OMe (CleanCap AG 3′OMe), or M6 3′OH and that this improvement is likely due at least in part to impaired Dcp2-mediated decapping. Thus, utilizing CleanCap M6 may help increase the potency of mRNA medicines, allowing for reduced dosing and increased efficacy in the clinic.
As a final comment, the authors indicated that future studies will explore the interplay between modified bases, including m1ψ, and modified caps, including M6 3′OMe, to determine whether additional synergistic combinations can provide even more potent effects on mRNA longevity.
Your comments are welcomed, as usual.
Please feel free to share this blog with your colleagues or on social media.